homework assignment help is most useful online help portal for the students that providing all Online s-p-d-f Block Elements assignment help Services.In the long form of the periodic table, elements are grouped into four main blocks, purely on the basis of electronic configurations. Elements are grouped in blocks 's', 'p', 'd' and 'f' depending on the nature of orbital(s) into which the last electron of the atom enters.
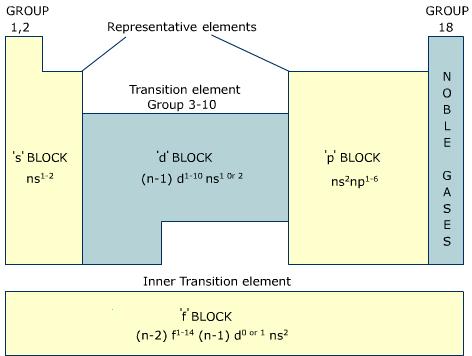
Location of the four blocks of elements s, p, d, f in the long form periodic table
's' block elements, (except helium) are those in which the valence-shell is the 'ns' orbital and where the last electron enters into the 's' orbital of the outer element. The general outer electronic configuration of such elements is either ns1or ns2. The 's' block elements belong to group 1 and 2 of the long form periodic table. They are situated on the left side of the table.
The main characteristics of the 's' block elements are:
![]()
![]()
'p' block elements are those in which the outer electronic configuration is of the type ns2 np1 to ns2 np6 and where the last electron enters into any of the outermost 'p' orbitals. These elements belong to groups 13 to 18 of the long form of periodic table and are situated on the right hand side of the table.
The main characteristics of the 'p' block elements are:
'd' block elements are those in which the added electron goes into one of the 'd' orbitals. These elements have valence electrons in both their outermost and penultimate shells (second outermost) and have a general outer electronic configuration of (n - 1) d1-10 ns1-2.
The penultimate shell in these elements is expanded from 8 to 18 by the inclusion of ten 'd' electrons. It is for this reason that these elements are called 'd' block elements. Thus, 'd' block elements are those, which in their elemental or combined forms have partially filled 'd' orbitals. They are also referred to as transition elements.
'd' block elements belong to groups 3 to 12 of the long form of periodic table and are situated in the middle of the table between 's' block and 'p' block elements.
All 'd' block elements are classified into four transition series, namely:
3d series in the 4th period having 10 elements
4d series in the 5th period having 10 elements
5d series in the 6th period having 10 elements
6d series in the 7th period having incomplete elements.
The main characteristics of the 'd' block elements are:
Because of their characteristic electronic configuration, the properties of transition elements ('d' block elements) in any period do not differ much from each other (unlike nontransition elements from the same period). The electronic configuration of transition elements can be written as: (n - 1) dx nsy where, x = 1 to 10 and y = 1, 2.
This indicates that:
The 'f' block elements are those, which in their elemental or ionic forms have partially filled 'f' orbitals. The differentiating (last) electron enters 'f' orbitals, which lie inner to the second outermost (penultimate) shell. Thus these elements are also known as inner-transition elements.
There are two series of 'f' block elements, each having 14 elements. Lanthanides (atomic number 58 - 71) are those inner-transition elements in which 4 'f' orbitals are progressively filled. Actinides (atomic number 90 - 103) are those elements in which 5 'f' orbitals are progressively filled.
The 'f' block elements are placed at the bottom of the long form of periodic table in the form of two rows. Most actinides are radioactive.
The general outer electronic configuration of 'f' block elements is, (n - 2) f1-14 (n - 1) d0-1 ns2
The main characteristics of the 'f' block elements are:
Elements can also be classified according to their chemical behaviour (as apart from electronic configurations and similar properties).
Homework Assignment Help is World No 1 Online Assignment Help Company
@2010 - Copyright ©2025 All rights reserved | This is made with by Homework Assignment Help self
In case you face any problem or have any query please email us at :- info[@]homeworkassignmenthelp.com
Submit us an Assignment:
For Demo Class Click hereRead more
Our tutors start working only after the payment is made, to ensure that we are doing work only for serious clients and also our solution meets the required standard.
Getting homework help was never so easy you just need to follow following steps:
(for example: EST, Australian GMT etc)
any example or format you want the solutions to be in.
send you the price quoted by our tutor along with the time needed to solve the assignment
In case you face any problem or have any query please email us at :- info[@]homeworkassignmenthelp.com