In a bond ,two atoms are bound by either donating-accepting or sharing of electrons.In a molecule like NaCl, the electronegativity difference between Na and Cl is quiet substantial,which allows the chlorineto 'snatch' the electron from sodium.Thus ionic bond is formed.
In a molecule having bond like C=O,the electronegativity of oxygen is greater than carbon,however not to that extent as would enable the to snatch the electron from .Thus the density of electrons in the bond is 'pulled ' towards oxygen giving rise to polar covalent bond.
In case of C-C bond,since there is no difference between the electronegativities of two atms,it is non-polar covalent bond.
The molecule formed by more than two atoms coming together to form new compound. The atoms come together and are connected or held together by bonds.
The bonds are composed of electrons from the constituent atoms. The electrons are contributed by the constituent atoms, one each. There are two major types of bondings. They are ionic bonding and covalent bonding
Let us consider an example of sodium chloride molecule. Sodium has an excess electron than required for the stable configuration. Similarly the chlorine atom has one less electron in its configuration than required for a stable configuration.
In addition to this, the electronegativity of chlorine is very high.the electronegativity of sodium is very low . This difference in the in electronegativities of the atoms makes one atom as donor and another as an acceptor. This difference in the electronegativities allows chlorine to snatch an electron from the sodium-atom. In other words, it is that sodium donates and chlorine accepts the electron. As a result, a bond is formed between chlorine and sodium atom. This bond is called as ionic bond. Another example of and ionic bond is KCl.
Unlike As mentioned above, in a molecule, two atoms may have no difference or very low difference in the electronegativities. As such, no atom can snatch an electron from the onother atom. However, they may still share the electrons. The bonds so formed are called Covalent bonds. Covalent bonding can be two types.
Polar
Consider the example of C=O bond. The electronegativity of oxygen is more than that of carbon. As such the electron density is pulled more towards oxygen and a polarity is built in the molecule. Such a bond is known as polar covalent bond.
However, when there is no such difference in the electronegativities, the electrons are shared equally and this one is known as non-polar bond.
Let us see about types of chemical bonding. The presence of 8 electrons in the outermost shell of their atoms is responsible for stability of the noble gases, therefore atoms of all elements tend to obtain 8 electrons in their outermost shell by losing/gaining electrons with other atoms and this is the reason of their chemical combinations.
Sigma Bonding
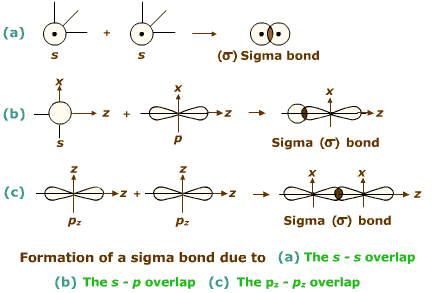
In the formation of ethane molecule, one of the hybridized bonds of each carbon atom chemical is used up in giving C-c bond and the remaining three bonds of each carbon atom are linked with hydrogen atoms. That means, each bond inethane is formed by the overlapping of orbital along their axes. Such bonds are known as sigma bonds.
Pi Bonding
In compounds of beryllium, boron and carbon, one pair of electrons is mutual between two atoms in the structure of a bond. In some compounds, particularly of carbon, two or three pairs of electrons are concerned in a bond which is the n known a double or a triple bond.
Covalent Compounds Bonding
In large number of compounds, atoms also mingle by sharing of the electronics in their outermost shells to inclusive their respective octets. This type of linkage is known called covalent bond. The compounds formed by this method known as covalent compounds bond.
Co-ordinate Compound Bonding
A different types of covalent linkage can be formed when both electrons for sharing between the two atoms are donated by one atom. This kind of linkage is called as coordinate bond.
Metallic Bonding
Metals normally have low ionization energies since the electrons in the outer shell can be taken out moderately easily. The force that binds a metal atom to a number of electrons within its sphere of power is called as a metallic bond.
Hydrogen Bonding
In composites of hydrogen with powerfully electronegative elements a such as fluorine, oxygen and nitrogen the electron pair shared between the two atoms fabrication so far away from the hydrogen nucleus that the latter becomes highly positive.
Two types of covalent bonds are formed depending on the way the two atomic orbitals overlap with each other.
When the overlap of orbitals of two atoms takes place along the line joining the two nuclei (orbital axis) then the covalent bond formed is called sigma (s) bond. These bonds can be formed due to 's-s', 's-p' or 'p-p' overlap along the orbital axis. Free rotation around a sigma bond is always possible.
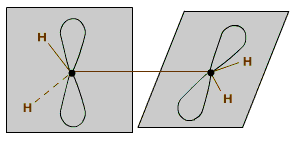
When the two atoms overlap due to the sideways overlap of their 'p' orbitals, the covalent bond is called as pi(p) bond. In a pi bond the electron density is concentrated in the region perpendicular to the bond axis.
Homework Assignment Help is World No 1 Online Assignment Help Company
@2010 - Copyright © All rights reserved | This is made with by Homework Assignment Help self
In case you face any problem or have any query please email us at :- info[@]homeworkassignmenthelp.com
Submit us an Assignment:
For Demo Class Click hereRead more
Our tutors start working only after the payment is made, to ensure that we are doing work only for serious clients and also our solution meets the required standard.
Getting homework help was never so easy you just need to follow following steps:
(for example: EST, Australian GMT etc)
any example or format you want the solutions to be in.
send you the price quoted by our tutor along with the time needed to solve the assignment
In case you face any problem or have any query please email us at :- info[@]homeworkassignmenthelp.com