The nuclear atomic physics is based on the structure of nucleus and the features of nucleus of an atom. The famous scientist, Rutherford, discovered the atomic nucleus in 1911 by the alpha particle scattering experiment. The nuclear atomic physics deals with the study of atomic nucleus. Rutherford found that the central core of every atom, which contains entire positive charge and almost all the mass of the atom, is called as nucleus. The size of atom is of the order of 10-10 m, whereas the size of nucleus is about 10-15 m. Therefore, he said that the most of the part of atom is empty. The phenomenons of the nucleus are – radioactivity, nuclear fusion and nuclear fission.
There are two types of radioactivity: one is natural radioactivity and other is artificial radioactivity. Natural radioactivity is a spontaneous and self-disruptive activity given by the number of heavy elements occurring in nature. Thus, radioactivity is the property by virtue of which a heavy element disintegrates itself without being forces by external agent to do so. The phenomenon of radioactivity is an atomic nuclear physics phenomenon discovered by the French scientist Henry Becquerel in 1896. He observed that the uranium salts possessed the particular property by which it affects the photographic plate. The phenomenon of emission of active radiations by an element was termed as radioactivity. The element showing such property is called radioactive element.
The phenomenon of a heavy nucleus splitting into two or more lighter nuclei thereby ejecting large amount of energy is called Nuclear fission . In the process of nuclear fission, some mass disappears. The difference in the mass is called mass defect and the energy associated by this mass defect is called as binding energy. Nuclear reactor is based on controlled nuclear fission.
Nuclear fusion is the phenomenon of fusing two or more lighter nuclei to from a single heavy nucleus. The mass of the product nucleus is slightly less than the sum of masses of lighter nuclei fusing together. This mass defect release lot of amount of energy. The Sun is the example of nuclear fusion.
Albert Einstein gave a famous equation to calculate the amount of energy ejected during the nuclear fission. According to his equation. 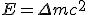 where
where 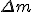 is the mass difference between the original atom and the split parts. So for energy to be released during the fission, the sum of masses of the split parts must be greater than the original one.
is the mass difference between the original atom and the split parts. So for energy to be released during the fission, the sum of masses of the split parts must be greater than the original one.
Homework Assignment Help is World No 1 Online Assignment Help Company
@2010 - Copyright © All rights reserved | This is made with by Homework Assignment Help self
In case you face any problem or have any query please email us at :- info[@]homeworkassignmenthelp.com
Submit us an Assignment:
For Demo Class Click hereRead more
Our tutors start working only after the payment is made, to ensure that we are doing work only for serious clients and also our solution meets the required standard.
Getting homework help was never so easy you just need to follow following steps:
(for example: EST, Australian GMT etc)
any example or format you want the solutions to be in.
send you the price quoted by our tutor along with the time needed to solve the assignment
In case you face any problem or have any query please email us at :- info[@]homeworkassignmenthelp.com