In organic chemistry, an Alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond.The simplest acyclic alkenes, with only one double bond and no other functional groups, form an homologous series of hydrocarbons with the general formula CnH2n.
Alkenes are unsaturated hydrocarbons containing a carbon double bond (C=C) in their molecule. They were earlier known as olefins.
Alkenes are described by the general formula CnH2n where
n = 1, 2, …..Alkenes are represented by the general formula,

R = alkyl group or H
The chemical behavior of alkenes is largely determined by the C = C functional group.
Various members of alkene family are: C2H4 Ethene (Ethylene) H2C=CH2
C3H6 Propene (Propylene) CH3-CH=CH2 C4H8 Butene (Butylene) CH3-CH3-CH=CH2 or CH3-CH=CH-CH3
Depending upon whether alkenes have an open chain or cyclic structure, they may be classified as acyclic or cyclic. A few examples of each class are:

Methane is the simplest carbon. Here, the four half filled orbitals belonging to the valence shell of carbon atom undergoes hybridization, resulting in the formation of four sp3 hybrid orbitals which are directed towards the corners of tetrahedron. Each of the sp3 hybrid orbitals of carbon overlaps with 1s orbital of hydrogen to form carbon-hydrogen sigma bonds. The carbon atom lies at the centre of the tetrahedron while the four hydrogens occupy the four corners or vertices of the tetrahedron.

Spatial formula of methane
In ethane there are two carbon atoms and six hydrogen atoms. Each carbon atom is sp3 hybridized. Thus, there are in all eight sp3 hybrid orbitals on the two carbon atoms. Two hybrid orbitals (one from each carbon) overlap with each other and form a C-C single bond. Other six hybrid orbitals (three on each carbon atom) form six C-H single bonds due to the overlap with the s orbitals of hydrogen atoms as shown.

The overlapping of two sp3 hybrid orbitals of the two carbon atoms, or of sp3 hybrid orbital of C and one s orbital of H atom, gives rise to a single bond called as sigma (s) bond.
In alkenes, two carbon atoms are bonded through a double bond in the parent chain. The formation of a double bond in alkenes is illustrated below by taking the simplest alkene, ethene, as a typical example.
Ethene can be described by the molecular formula C2H4. Each carbon atom in this molecule has three points of attachment and therefore, each carbon atom in ethene is sp2 hybridized.

 The sp2 hybridization of carbon orbital
The sp2 hybridization of carbon orbital
The three sp2 hybrid orbitals lie in a plane and are inclined to each other at an angle of 120° (see above figure). One of the p orbitals (say 2pz) on each carbon atom is left unhybridized.
The three sp2 hybrid orbitals form three sigma (s) bonds: two with H-atoms and one with the adjacent carbon atom. This results in a planar structure. The two partially filled unhybridized p orbitals of the two carbon atoms overlap side-ways to form a pi bond as shown:



Side-ways overlap of unhybridized pz orbitals of the two carbon atoms leading to the formation of a p bond

Simplified representation of ethene molecule
Therefore, in ethene,the carbon-carbon double bond (=) is a combination of a sigma (s) and a pi bond(p). The simple structural formula of ethene is written as shown above.
In alkynes, the two carbon atoms in the parent chain are bonded through a triple bond. The formation of triple bond in alkynes is illustrated below by taking ethyne (acetylene) as a typical example.
Ethyne (acetylene) can be described by the molecular formula C2H2. As each carbon atom in this molecule has two points of attachments, each carbon atoms in ethyne (acetylene) is sp hybridized.
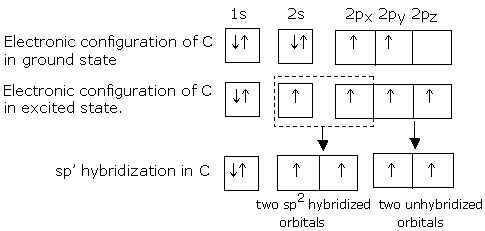

sp hybridization of carbon orbitals
The two 'sp' hybrid orbitals are oriented at an angle of 180° to each other, while the two p orbitals (py and pz) on each carbon are left unhybridized. These orbitals are at right angles to each other as well as to the sp hybrid orbitals.
One of the 'sp' hybrid orbitals overlaps with the 'sp' hybrid orbital of the other carbon atom to form a sp-sp sigma bond. The remaining two sp hybrid orbitals of the two carbon atoms overlap with the s orbitals of two hydrogen atoms to form two sigma bonds. The unhybridized py and pz orbitals of two carbon atoms overlap laterally to form two p bonds. The combination of one (sp-sp) sigma bond, and two (p-p) p bonds gives rise to the triple (  ) bond. Two p-electron clouds merge into each other to form a cylindral p-p electron cloud.
) bond. Two p-electron clouds merge into each other to form a cylindral p-p electron cloud.


Formation of a triple bond between two carbon atoms due to sideways overlap of the unhybridized orbitals

Homework Assignment Help is World No 1 Online Assignment Help Company
@2010 - Copyright © All rights reserved | This is made with by Homework Assignment Help self
In case you face any problem or have any query please email us at :- info[@]homeworkassignmenthelp.com
Submit us an Assignment:
For Demo Class Click hereRead more
Our tutors start working only after the payment is made, to ensure that we are doing work only for serious clients and also our solution meets the required standard.
Getting homework help was never so easy you just need to follow following steps:
(for example: EST, Australian GMT etc)
any example or format you want the solutions to be in.
send you the price quoted by our tutor along with the time needed to solve the assignment
In case you face any problem or have any query please email us at :- info[@]homeworkassignmenthelp.com